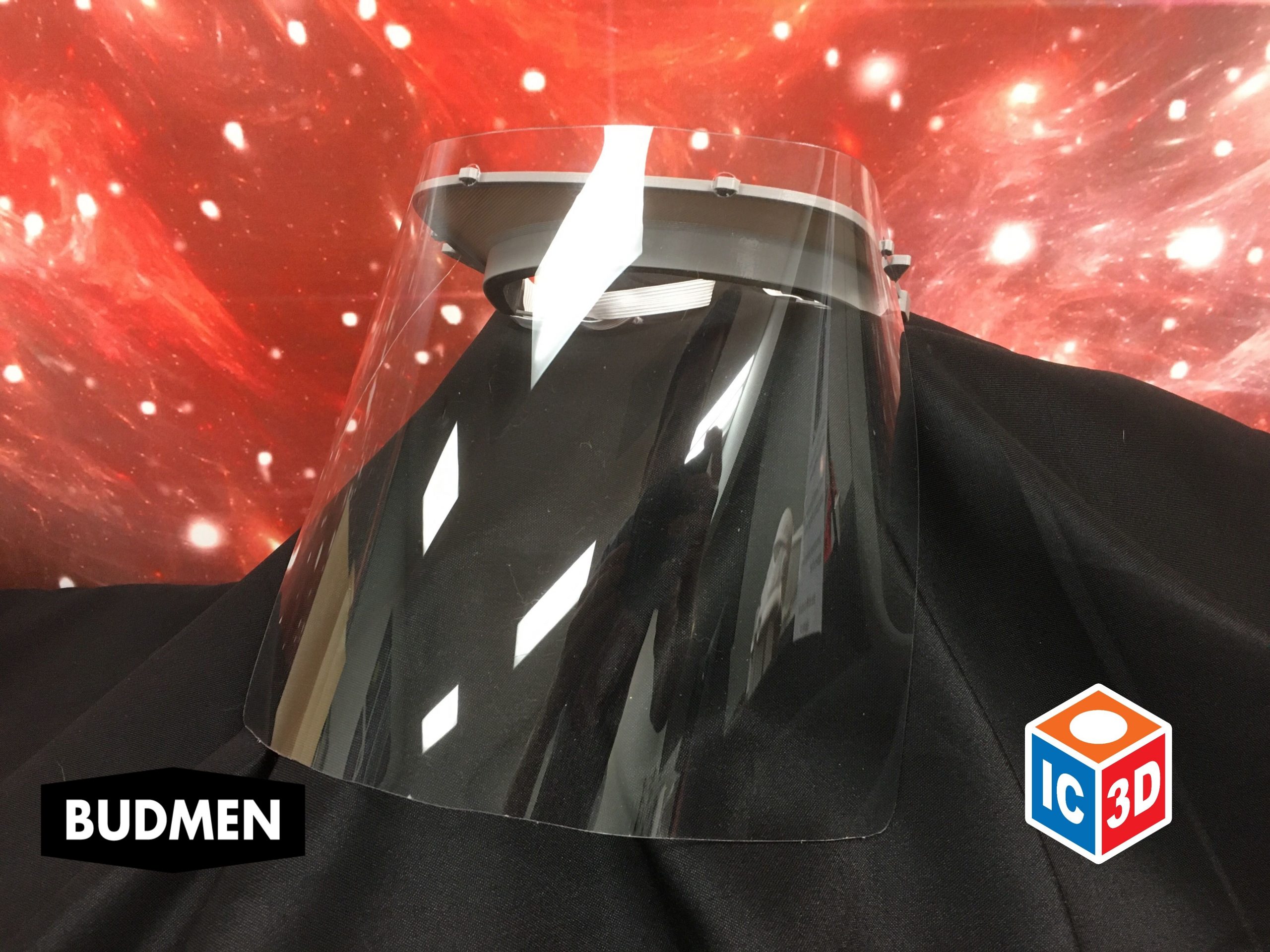
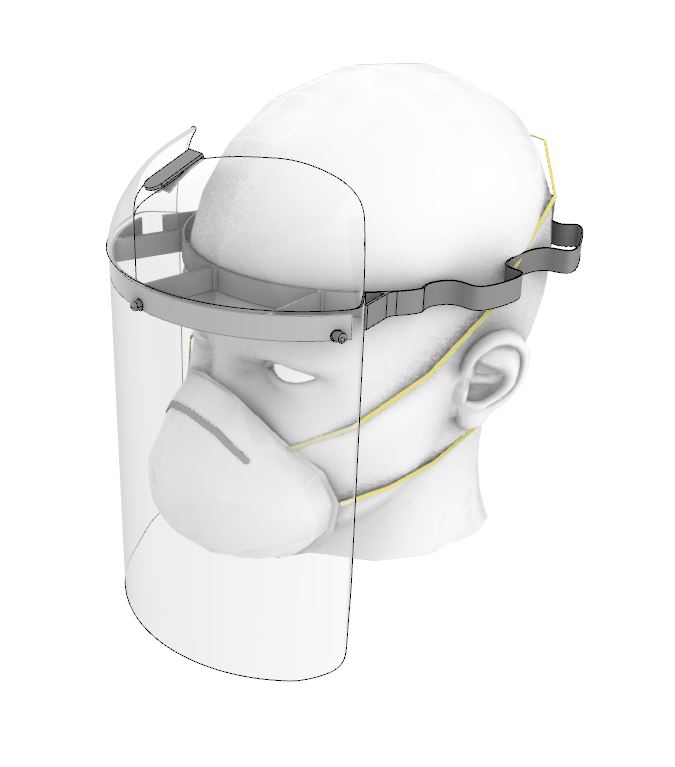
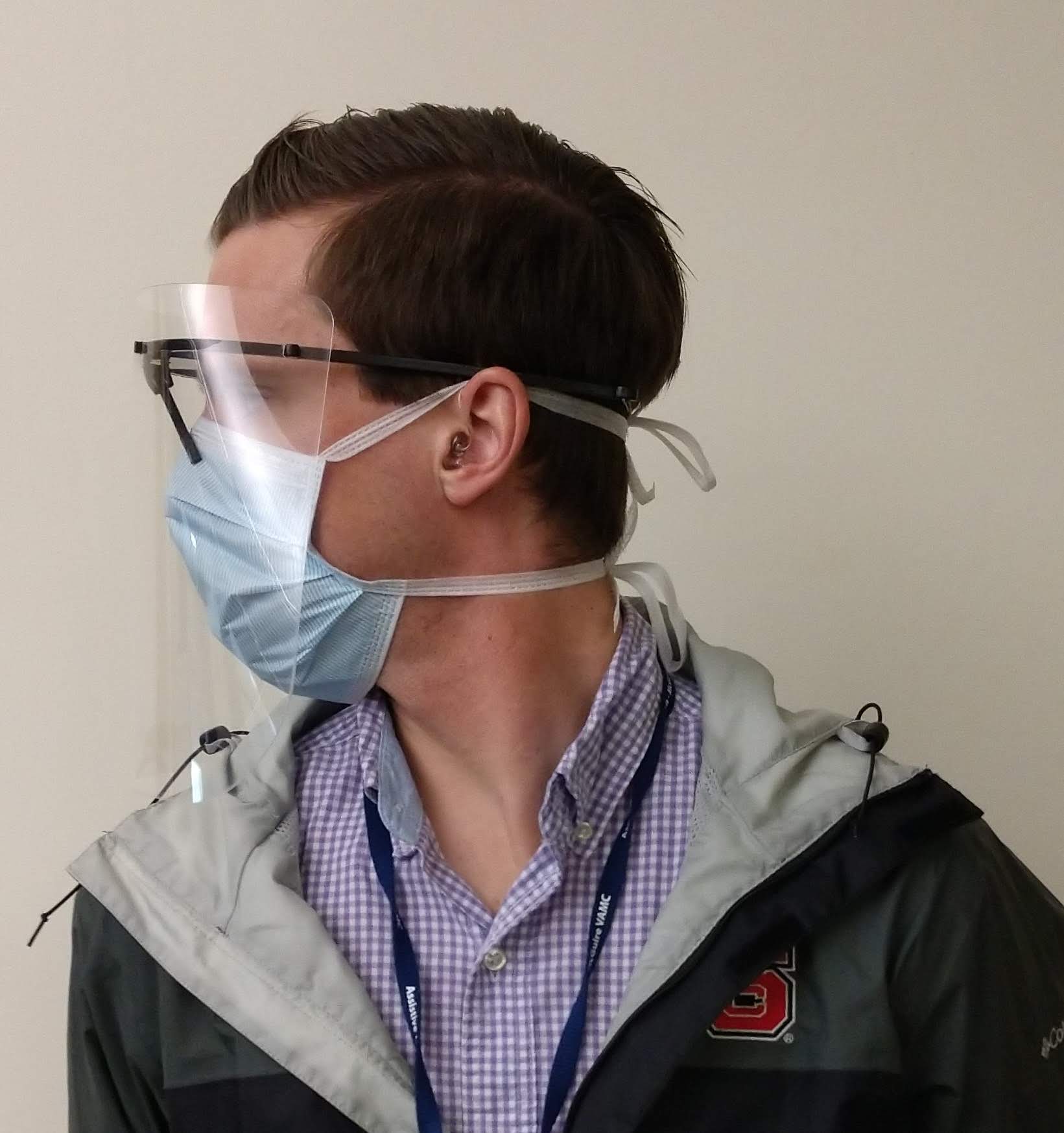
Patients with COVID-19 experience significant respiratory issues, resulting in coughing. Virus particles are easily spread in the fluids expelled from the patient during episodes of coughing. The face shield is an additional barrier between the healthcare worker and the patient and reduces the risk of viral transmission via airborne droplets.